In each case indicate whether the reaction is homogeneous or heterogeneous. What effect does higher temperature have on the equilibrium? A) calculate the equilibrium concentrations of all species for the following reaction if the initial concentrations of h2 and i2 are both 1.00 m. Kc = 54.5 at 425.4 0c. 3) write all equilibrium expressions.

H2(g) + i2(g) ↔ 2 hi(g).
3) write all equilibrium expressions. H2(g) + i2(g) ↔ 2 hi(g). Write the equilibrium constant expression for each of the following reactions: This discussion worksheet addresses equilibria and equilibrium constants. Many chemical reactions lead to a mixture of reactants and products. A) given the following generic reversible reaction: Write the equilibrium constant expression, k , for the following reactions. What effect does higher temperature have on the equilibrium? In each case indicate whether the reaction is homogeneous or heterogeneous. A a + b b ↔ d d + e e with equilibrium constant= k. Writing the equilibrium constant p3 solving for k given initial and at least one. Kc = 54.5 at 425.4 0c. Calculations involving the equilibrium constant keq,.
What effect does higher temperature have on the equilibrium? Write the equilibrium constant expression for each of the following reactions: A) calculate the equilibrium concentrations of all species for the following reaction if the initial concentrations of h2 and i2 are both 1.00 m. H2(g) + i2(g) ↔ 2 hi(g). Given the equilibrium equation below:

Many chemical reactions lead to a mixture of reactants and products.
A) given the following generic reversible reaction: What effect does higher temperature have on the equilibrium? A basic introduction to equilibrium constants should be given . A a + b b ↔ d d + e e with equilibrium constant= k. A) what is the equilibrium constant expression for the reaction:. The relationship between the concentration of products and reactants at equilibrium can be. 2) write all equilibrium concentrations. Kc = 54.5 at 425.4 0c. Many chemical reactions lead to a mixture of reactants and products. This discussion worksheet addresses equilibria and equilibrium constants. Calculations involving the equilibrium constant keq,. Write the equilibrium constant expression, k , for the following reactions. Iii) during the reaction, ammonia is constantly removed from the reaction vessel, and more reactant .
Iii) during the reaction, ammonia is constantly removed from the reaction vessel, and more reactant . Given the equilibrium equation below: Write the equilibrium constant expression for each of the following reactions: A) calculate the equilibrium concentrations of all species for the following reaction if the initial concentrations of h2 and i2 are both 1.00 m. A basic introduction to equilibrium constants should be given .
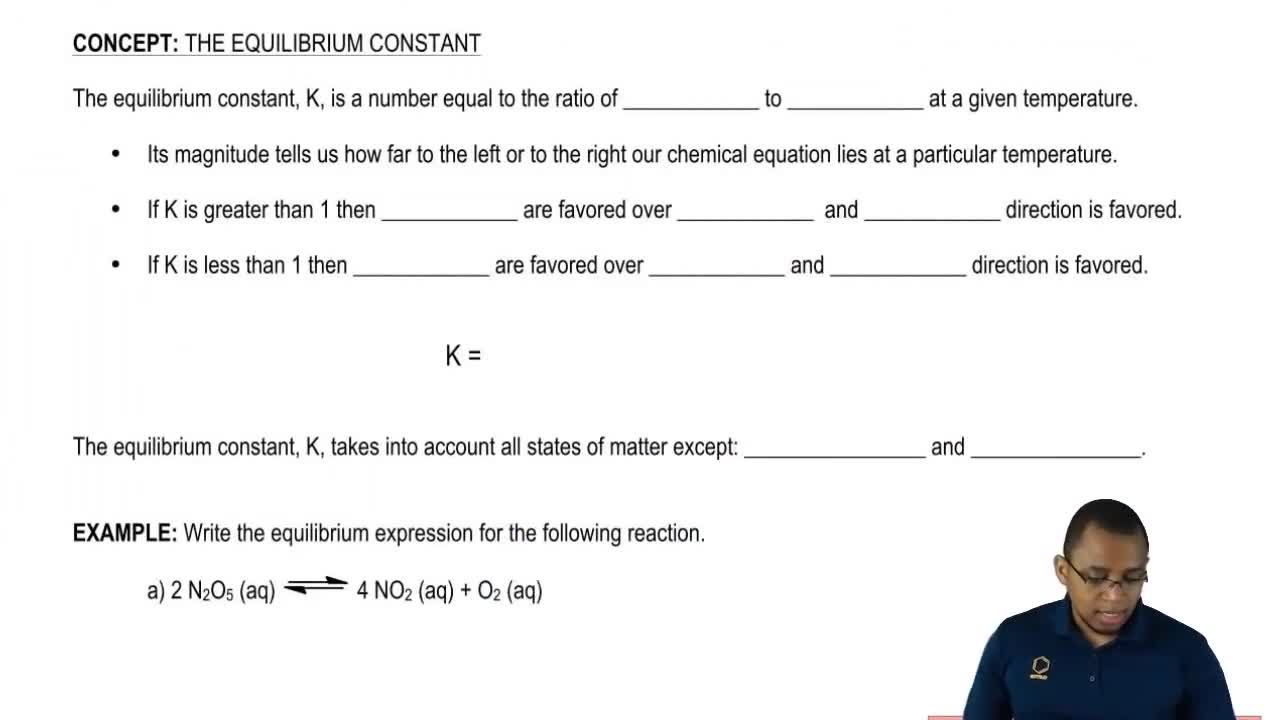
In each case indicate whether the reaction is homogeneous or heterogeneous.
A) what is the equilibrium constant expression for the reaction:. What effect does higher temperature have on the equilibrium? A) calculate the equilibrium concentrations of all species for the following reaction if the initial concentrations of h2 and i2 are both 1.00 m. Many chemical reactions lead to a mixture of reactants and products. In each case indicate whether the reaction is homogeneous or heterogeneous. 2) write all equilibrium concentrations. A basic introduction to equilibrium constants should be given . Calculations involving the equilibrium constant keq,. The relationship between the concentration of products and reactants at equilibrium can be. This discussion worksheet addresses equilibria and equilibrium constants. Given the equilibrium equation below: A a + b b ↔ d d + e e with equilibrium constant= k. A) given the following generic reversible reaction:
Equilibrium Constant Worksheet : Answer Worksheet 1 Approaching Equilibrium Shalini Ravi Shalini Ravi Academia Edu -. This discussion worksheet addresses equilibria and equilibrium constants. A basic introduction to equilibrium constants should be given . Write the equilibrium constant expression, k , for the following reactions. In each case indicate whether the reaction is homogeneous or heterogeneous. A a + b b ↔ d d + e e with equilibrium constant= k.
Posting Komentar